What Is the Molarity of Hcl
HCL 37 122 Molar. Molarity molL important formula for concentration Plug your values in.

Hess S Law Chemical Changes Physical Chemistry Chemistry
26 Is molarity the same as concentration.

. Dividing the grams of HNO 3 by the molecular weight of HNO 3 6301 gmole gives the number of moles of HNO 3 L or Molarity which is 157 M. 1 liter 1185 gm 444 gm HCl 375 122 moles range 1185 1234 Boiling Point 110C 230F. If we work at STP 1 mol HCl gas has volume 224 L.
What is the molarity of 12N HCl. The following equation is used for calculating acid and base molarity where the concentration is given in wt. Calculate the molarity of 30 wW NaOH solution if the density of the solution is 105 gcc.
Now 000325 mol H Cl 0030 L 00108M H Cl. D MW 10 Molarity. Molarity M Normality N Volume mL required to make 1000 mL solution.
Weight of HCl in 1000 ml of solution 06909 x 1000 6909 g. Now the question is - how many moles HCl gas are in 100 cm³ volume. HCl to 1L of water or 83ml to 100ml.
Therefore weight of HCl in 1ml of solution specific gravity x 49 141 x 49100 6909 gml. 37 ww hydrochloric acid means 37 g hydrogen chloride is present in 100 g solution. When you substitute 1014 mol HCl and 0084 L into the molarity equation we find that the molarity is around 1207 M HCl which is the highest concentration of HCl that we can obtain at room temperature.
Add 2mol12M 167 ml conc. In addition in the case of HCl 1N1M. Therefore we can say that 1 liter of Hydrochloric acid contains 12178 moles of HCl or in other words molarity of 37 ww Hydrochloric acid is equal to 12178 M.
22 What is the numerical value of mole. For the hydrochloric acid it is equal to 3646 gmol. Molarity M is the amount of a substance in a certain volume of solution.
Hence the molarity of HCl solution is 0262 M. To calculate the molarity of solution we use the equation. What is the molarity of a solution containing 170 g of NH3 in 120 l of solution.
Therefore in 100 cm³ HCl gas you have 100 cm³ 224 L 1000 cm³L 000446 mol HCl gas dissolved in 10 L solution. What is the equation used for Molarity Conversion. Given mass of HCl 03366 g.
Multiply 100 g of our HCl solution by our density conversion factor of 1 mL119g to get 84 mL and then divide by 1000 to get 0084 L. Molarity weight of HCl in 1000 ml molweight of HCl 6909364 189 M. In the acids context Normality N refers to the hydroxide ions that are released in the water when the acid is dissolved.
What is the molar concentration of HCl. Molarity dfractextweight of HCl in 1000 mltextMolecular weight of HCldfrac69093641892M Molecular weight of the HCl 364 - Therefore the molarity of a solution of HCl which contains 49 by weight of. Since molarity refers to number of moles in 1000 ml solution calculate the weight of 1000 ml hydrochloric acid using the following formula.
23 What will be the molarity of a solution containing 1825 gram of HCl 500 grams of water. The molar mass of HCl is 36461 gmol so 37088 g HCl 1 mol HCl36461 g HCl 10172 mol HCl in 1 L of solution. Lets assume that it is the hydrochloric acid HCl.
Decide on the mass concentration of your substance you can either input it directly or fill in the boxes for substance mass and solution volume. The molar mass of hydrogen chloride is 3646 gmol. 000325 mol H Cl.
0025 L of 013 mol L N aOH 000325 mol N aOH. What is the molarity of 37 HCl. Molarity is defined as the moles of a solute per liters of a solution.
24 What will be the molarity of a solution containing 585 g of NaCl 500ml solution. Molarity 000446 mol L HCl. Add 1mol12M 83 ml conc.
Molarity of liquid HCl with density equal to 117gcc is. H Cl N aOH H 2O N aCl. Find the molar mass of your substance.
The equation for the reaction is NaOH HCl NaCl H2O My solution 100 mLx molarity 3234 mL0108 molarity x molarity 0349 Does it look right. In the stomach however the solution becomes diluted to a pH between 1 and 2 which corresponds to a hydrochloric acid concentration between 01 and 001 moles per liter. 10172 mol HCl1L solution 10172 M HCl.
Volume of the solution 3523 mL. Molar mass of HCl 365 gmol. Strength 365-38 Density 1185 Molecular Weight 365.
1317M - but you used two significant figures in the question so. A 100 mL sample of aqueous HCl requires 3234 mL of 0108 molarity of NaOH to reach the endpoint. Molarity is also known as the molar concentration of a solution.
Putting values in above equation we get. 12g HCl 1mol HCl3646g HCl 0329 mol HCl. 25 What will be the molarity of a solution containing 5 g.
21 Is molarity moles per liter. What Is The Mass Percent Of Chlorine In Hydrochloric AcidThe mass of Cl in HCl is 9726What is the percent by mass of chlorineThe mass percentage of chlorine is 6066 chlorine in the compound sodium chlorideIs there chlorine in HClhydrogen chloride HCl a compound of the elements hydrog. Given 49 weight by volume HCl solution.
How do you make a 2 solution of HCl. Molar mass of HCl 101gmolar mass of H 3545gmolar mass of Cl 3646g HCl. 000325 mol N aOH 1 mol H Cl1 mol N aOH.
The molarity of HCl solution is 0262 M.

Welcome To Learnapchemistry Com Ap Chemistry Ap Chem Question Paper

Molarity Ph Chemistry Education Chemistry Lessons Teaching Chemistry

Molarity Worksheet Chemistry Worksheets Teaching Chemistry Chemistry Lessons

Ap Chemistry Problem Of The Day Ap Chemistry Chemistry Classroom Science Chemistry

Molarity And Molality Calculations High School Chemistry Chemistry Teaching

Ncert Solutions For Class 11 Chemistry 11th Chemistry Chemistry Solutions

Titration Practical And Calculation Naoh And Hcl Teaching Chemistry Chemistry Lessons Chemistry Practical

Ionization Energy Ionization Energy Electron Configuration Chemistry

Heat Of Combustion Physical Chemistry Chemistry Chemical Equation

Neet 2018 Qp With Solutions Code Aa Part 18 Question Paper Writing A Cover Letter Math Questions

Molarity Ph Teaching Chemistry Chemistry Education Chemistry Lessons

Ncert Solutions Ncertsolutionsforclass12 Ncertsolutionsforclass12chemistry Ncertsolutionsforclass12chemistrychapter2solution In 2021 Chemistry Solutions Molar Mass

Welcome To Learnapchemistry Com Ap Chemistry Teaching Chemistry Chemistry Classroom
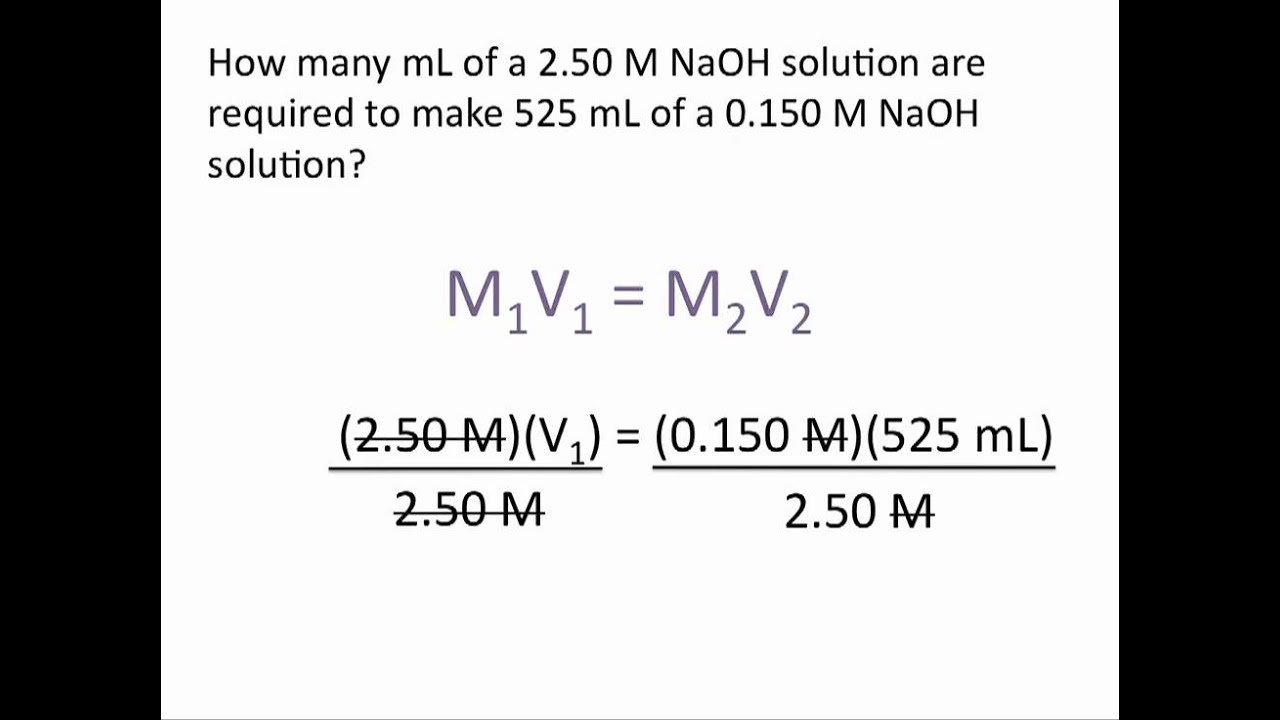
Dilution Problems Chemistry Tutorial Chemistry Worksheets Chemistry Classroom Chemistry Education

Trick To Calculate The Ph From Molarity And Normality Youtube Physical Chemistry How To Find Out Net Exam

Molarity 2 Molarity M This Is The Most Common Expression Of Concentration M Molarity Moles Of Solute Mol Liter Study Chemistry Biology Notes Solutions

Molarity Practice Worksheet Answer New Molarity Practice Worksheet Answers Ch Word Problem Worksheets Scientific Notation Word Problems Word Problem Practice

What Is Stoichiometry And Why Is It Used In Chemistry A Plus Topper Whatisstoichiometry Chemistry Chemistry Education Stoichiometry Chemistry

Comments
Post a Comment